Title: PREPREGS AND MOLDINGS PRODUCED THEREFROM
Number/Link: US2014065911
Applicant/Assignee: Evonik
Publication date: 6-03-2014
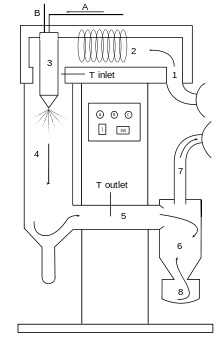
“Gist”: A reactive polyurethane powder is prepared from a mixture of a solid polyester polyol and a solid dimerised IPDI and subsequently used to make glass fiber prepregs.
Why it is interesting: A polyurethane powder is made from (pref.) a mixture of a solid (at room temperature) polyster polyol and a solid, blocked isocyanate. The isocyanate is preferably an “internally” blocked IPDI. Internally blocked meaning that the isocyanate contains uretidinedione groups. The powder is scattered over a fibrous support and heated to over melting temperature but below curing temperature to make the prepreg. Prepregs made with PU powder instead of the conventional resins have the advantage of being non-sticky, non-toxic and of having a very high storage stability (45 days in the examples).