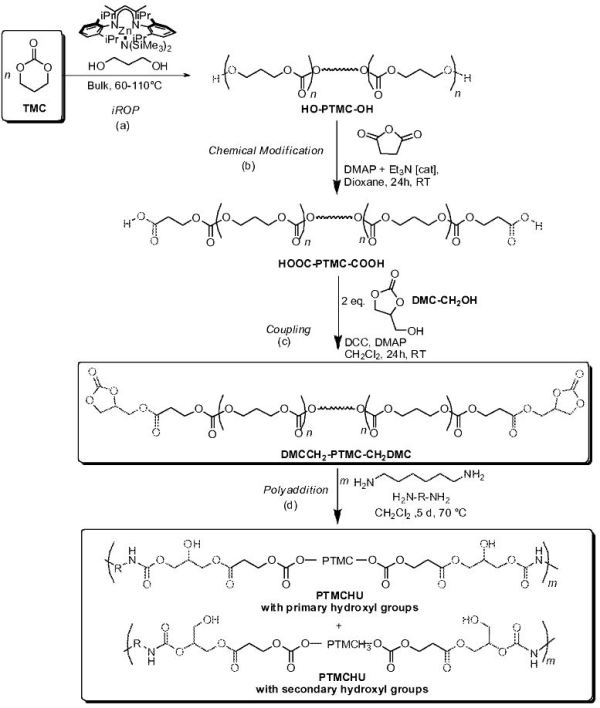
Title: METHOD FOR PREPARING POLY(CARBONATE-URETHANE) OR POLY(ESTER-URETHANE)
Number/Link: US20130144027
Applicant/Assignee: CENTRE NAT RECH SCIENT; TOTAL RES & TECHNOLOGY
Publication date: 6-06-2013 (priority PCT/EP)
“Gist”: Non-isocyanate polycarbonate- or polyester- polyurethane with very high softblock molecular weight produced by “immortal” ring opening polymerization.
Why it is interesting: Non-isocyanate PU systems appear to become a growing trend – at least in scientific and patent literature. In addition to not using phosgene or isocyanates, advantages quoted are improved biodegradability and recyclability. The current invention teaches the use of “immortal” ringopening polymerization to polymerize 5,6 or 7 membered cyclic carbonates or cyclic esters. This type of ROP is highly efficient and is described in e.g. US2011092664. The resulting polymer is subsequently modified with anhydrides to a polymer with carboxylic end-groups, which are then reacted with cyclic carbonates bearing hydroxyl groups. The resulting polymer has a MW of (preferably) 50,000 to 100,000 and can be reacted with polyamines to produce the polyurethanes. See the reaction scheme below.